
“The series of eleven n-heptane/air flames demonstrate the transition that occurs in a turbulent flame as a result of low temperature oxidation of the reactants prior to their introduction into the high temperature flame. Scanning left to right, the degree of pre-flame reactant oxidation is increased by increasing the reactant temperature and/or the heated residence time. This transition, evident by the blossoming redness of the flames, can have serious implications on the flame properties, including burning rates, emissions, turbulent/combustion interactions, and flame regimes.”
Bret Windom, Bo Jiang, Sang Hee Won, Yiguang Ju (Princeton University)
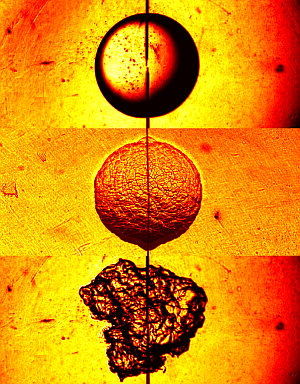
“The three images are snap shots of a spark-ignited expanding flame in different environments of the same hydrogen-air mixture.
The top flame shows the ideal, reference case of a stable, smooth flame surface in a quiescent environment at atmospheric pressure.
The middle flame is taken under elevated pressure simulating that within an internal combustion engine. The flame surface is now hydrodynamically unstable (i.e. the Darrieus-Landau instability) and develops cells, causing the flame to propagate and hence burn faster because of the increased flame surface area.
The bottom flame is taken in a highly turbulent environment which simulates another aspect of the engine interior. Now the flame surface is distorted by the multi-scale turbulence, which again leads to an increase of the burning rate. All images were taken at 8000 frame per second, using schlieren photography. Radius of the top flame is 11.4 mm.”
Chung K. Law, Swetaprovo Chaudhuri, Fujia Wu (Princeton University)
“The image is produced by simulating the combustion of a single coal particle in laminar flow using detailed chemistry.
The image shows the carbon monoxide (CO) concentration in the gas phase.
The blade corresponds to production of CO by devolatilization of the coal particle, the guard corresponds to homogeneous ignition and the grip corresponds to CO production by char oxidation.”
Babak Goshayeshi, James C. Sutherland (University of Utah)
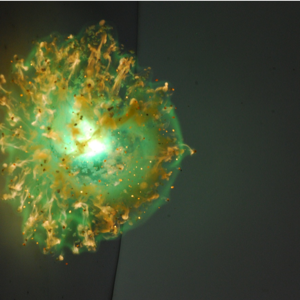
“This movie shows the hypergolic ignition of three solid compounds with concentrated nitric acid. Reaction begins within 10 ms and is completed in a fraction of a second. The green color indicates the presence of boron.”
M. Pfeil, J. Dennis, T. Pourpoint, S. Heister, P. Ramachandran, and S. Son (Purdue University)
“Copper oxide (CuO) nanowires grown on copper substrate by thermal annealing method are decorated with cobalt oxide (Co3O4) nanoparticles by burning cobalt precursor coated CuO nanowires for 10 sec in a CH4/air premixed flame (fuel lean condition). A high temperature and ultra-fast heating rate of the flame enables rapid combustion of cobalt precursor in the vicinity of the CuO nanowires (localized combustion), in which generated gaseous products blows out the precursor as it nucleate/crystallize, finally forms nanoparticles chains around CuO nanowires.”
In Sun Cho (Stanford University)